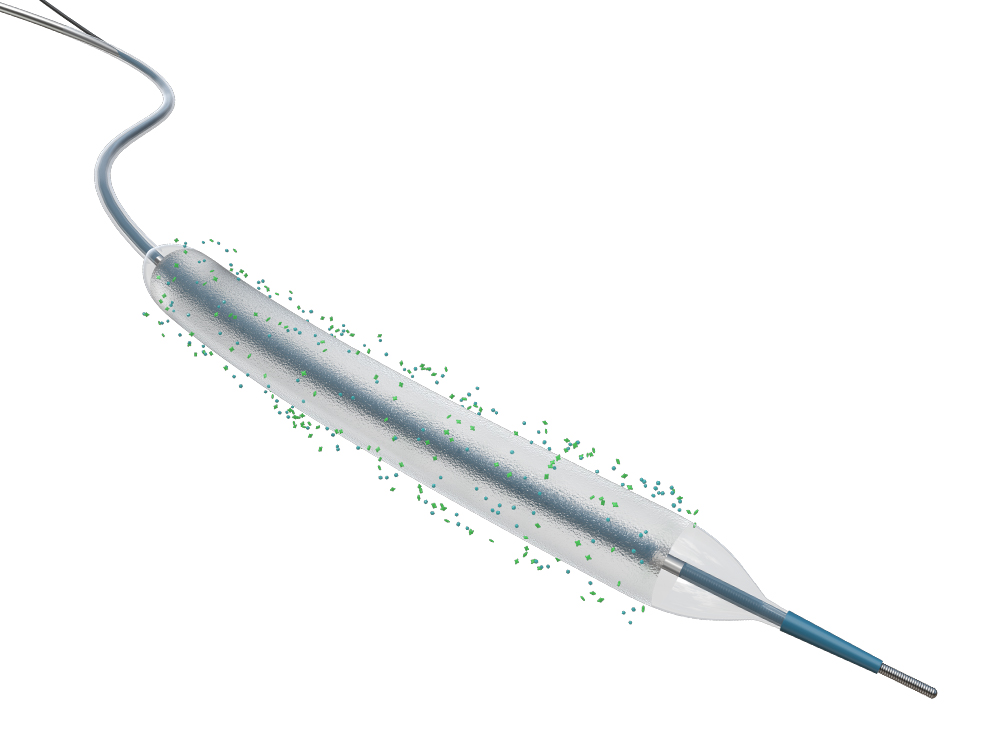
Vesselin® Drug Coated Coronary Balloon Catheter
Vesselin®
Vesselin® Drug Coated Coronary Balloon Catheter Details
Product Description
Indications:
• Recommended for the treatment of stent restenosis, small vessel disease, and branch lesions.
• Clinical evidence supports primary coronary artery lesions such as macrovascular lesions.
Materials
• Improved PEBAX guarantees higher flexibility and enhanced crossability
Drug
• Loaded Paclitaxel inhibits restenosis continuously.
Drug Coating
• Coated with Active Pharmaceutical Ingredients grade urea, provide better biocompatibility, facilitate drug-releasing effectively.
Delivery system
• Spiral cut hypotube design, strengthen the transition section, bring optimized deliverability for transition.
Vesselin®
Vesselin® Drug Coated Coronary Balloon Catheter Details
Product Description
Drug Coating
Coating with Active Pharmaceutical Ingredients grade urea, provide better biocompatibility, facilitate drug-releasing electively. Drug release rate in vivo achieves more than 90%.
The suitable particle size at 2-3µm, enables long term efficacy and low embolism risk
Drug
Paclitaxel loaded with drug dose at 3µg/mm², inhibits restenosis continuously
Delivery System
Spiral cut hypotube design, strengthens the transition section, brings optimized deliverability for transition.
Materials
Improved PEBAX material, lower crossing profile guarantees higher flexibility and enhanced crossability.
Technique Parameters
Balloon Material: PEBAX
Euective length: 1350mm
Distal Shaft O.D.: 2.7F
Balloon crossing profile: 0.026” – 0.035”
Guiding catheter compatibility: 5F and above
Guide wire compatibility: 0.014”
Balanced safety and effectiveness
Compared with the control group, no significant difference in late lumen loss in
the segment in 9 months, thrombosis and TLF in 12 months.(TLF: Target lesion failure)



Drug balloon manipulation procedures instruction

Ordering Information
| Diameter (mm) | Length (mm) | ||||||||||
| 9 | 12 | 14 | 16 | 18 | 20 | 24 | 28 | 31 | 35 | 38 | |
| 2.0 | LPCDB 20009 | LPCDB 20012 | LPCDB 20014 | LPCDB 20016 | LPCDB 20018 | LPCDB 20020 | LPCDB 20024 | LPCDB 20028 | LPCDB 20031 | LPCDB 20035 | LPCDB 20038 |
| 2.5 | LPCDB 25009 | LPCDB 25012 | LPCDB 25014 | LPCDB 25016 | LPCDB 25018 | LPCDB 25020 | LPCDB 25024 | LPCDB 25028 | LPCDB 25031 | LPCDB 25035 | LPCDB 25038 |
| 2.75 | LPCDB 27009 | LPCDB 27012 | LPCDB 27014 | LPCDB 27016 | LPCDB 27018 | LPCDB 27020 | LPCDB 27024 | LPCDB 27028 | LPCDB 27031 | LPCDB 27035 | LPCDB 27038 |
| 3 | LPCDB 30009 | LPCDB 30012 | LPCDB 30014 | LPCDB 30016 | LPCDB 30018 | LPCDB 30020 | LPCDB 30024 | LPCDB 30028 | LPCDB 30031 | LPCDB 30035 | LPCDB 30038 |
| 3.5 | LPCDB 35009 | LPCDB 35012 | LPCDB 35014 | LPCDB 35016 | LPCDB 35018 | LPCDB 35020 | LPCDB 35024 | LPCDB 35028 | LPCDB 35031 | LPCDB 35035 | LPCDB 35038 |
| 4.0 | LPCDB 40009 | LPCDB 40012 | LPCDB 40014 | LPCDB 40016 | LPCDB 40018 | LPCDB 40020 | LPCDB 40024 | LPCDB 40028 | LPCDB 40031 | LPCDB 40035 | LPCDB 40038 |
| 4.5 | LPCDB 45009 | LPCDB 45012 | LPCDB 45014 | LPCDB 45016 | LPCDB 45018 | LPCDB 45020 | LPCDB 45024 | LPCDB 45028 | LPCDB 45031 | LPCDB 45035 | LPCDB 45038 |
Specifications
| Technical Parameters | |
| Distal Shaft O.D | 2.6F |
| Minimum Guiding Catheter Compatibility | 5F |
| Guide wire Compatibility | 0.014″ |
| Effective Length | 1350mm |
| Nominal Pressure | 8 atm(Φ2.0mm-Φ3.0mm)/ 6 atm(Φ3.5mm-Φ4.5mm) |
| Rated Burst Pressure | 16 atm(Φ2.0mm-Φ3.5mm)/14 atm(Φ4.0mm-Φ4.5mm) |